Scientists have made an advancement in the field of electrocatalysis. Their latest research, published in the journal Nature Materials, sheds light on how catalysts can stay in unanticipated forms during the process of nitrate reduction.
The study, titled “Revealing Catalyst Restructuring and Composition During Nitrate Electroreduction through Correlated Operando Microscopy and Spectroscopy,” offers new insights that could pave the way for more efficient catalyst design. The study is a collaboration between researchers from the Interface Science Department of the Fritz Haber Institute of the Max Planck Society in collaboration with beamline scientists at the Helmholtz-Zentrum Berlin.
Catalysts are substances that speed up chemical reactions without being consumed in the process. They are crucial in many industrial applications, from producing fuels to manufacturing pharmaceuticals.
However, understanding how these catalysts behave while they are working has always been a challenge. This is because catalysts can change their structure (size and shape) and composition when an electric potential is applied, much like how a chameleon changes its color to blend into different environments. A long-standing assumption is that, also like the chameleon, the catalyst will quickly transform into its preferred state (active state) once the electrical potential is applied.
A multi-modal approach to study catalysts
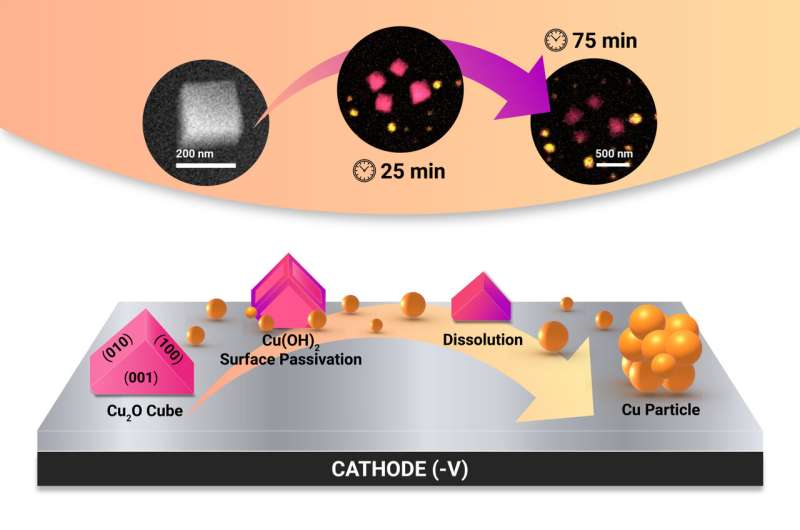
The research team employed a unique combination of advanced techniques to show that this assumption is not valid under certain conditions. First, they used a method called electrochemical liquid cell transmission electron microscopy (EC-TEM) to follow cubic Cu2O pre-catalysts under conditions where they were participating in the nitrate reduction reaction that is here being utilized to generate green ammonia.
This technique allowed them to see how the catalysts, specifically cubic Cu2O pre-catalysts, changed during the reaction. Then, they used a combination of X-ray microscopy/spectroscopy and Raman spectroscopy to check whether the pre-catalysts transform into the expected Cu metal phase during reaction, and whether such transformation was homogeneous over all nanocatalyst particles.
A significant finding of the study is that the Cu2O cubes do not quickly turn into the preferred metallic state and can stay as a mix of Cu metal, Cu oxide and Cu hydroxide for a long time during operation. The composition of this mixture and the shape of the evolved catalysts depend heavily on the electric potential applied, the surrounding chemical environment and the reaction duration.
Implications for ammonia selectivity
A big motivation for studying nitrate reduction is to explore its potential for recycling waste nitrates by turning them back into ammonia, a key ingredient in fertilizers for food production. So far, our strategies for optimizing this process have been based on expecting catalysts to adopt their most favorable forms during reaction. This research will pave the way towards new ways to design the Cu-based pre-catalysts that are better at producing ammonia.
Dr. See Wee Chee, a group leader at the Interface Science Department and corresponding author of the study emphasizes, “It is unexpected that we get different phases during reaction especially when we start from a single form of a single element pre-catalyst. More importantly, this mixed state can be maintained for a long time, which is valuable insight if we want to design more efficient catalysts.”
This research also demonstrates how advanced, real-time observation techniques that can capture local chemical differences can help us understand the complex nature of catalysts at work.
Prof. Beatriz Roldán, director of the Interface Science Department at the FHI and co-corresponding author stated, “Industrially, NH3 is synthesized via the gas-phase Haber-Bosch thermal catalysis method, which takes place at moderate temperatures (450–550 °C) but high pressures (150 bar) with a large consumption of fossil-generated H2.
“The challenge we tackled here was to find an alternative method for NH3 synthesis with reduced carbon emissions. This was accomplished by following a direct electrocatalytic route driven by renewable electricity.”
More information:
Aram Yoon et al, Revealing catalyst restructuring and composition during nitrate electroreduction through correlated operando microscopy and spectroscopy, Nature Materials (2025). DOI: 10.1038/s41563-024-02084-8
Provided by
Max Planck Society
Citation:
The secret life of catalysts: Scientists reveal unanticipated forms during nitrate reduction (2025, January 29)
retrieved 29 January 2025
from https://phys.org/news/2025-01-secret-life-catalysts-scientists-reveal.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.