Scientists from The University of Manchester have developed a new material that could help reduce water pollution caused by harmful chemicals, such as from leftover medicines and hygiene products, that end up in rivers and lakes.
Water pollution is one of the growing challenges of modern life. Many everyday items, from medications to cosmetics, leave behind residues that don’t fully break down after use. These pollutants often find their way into water systems, where they disrupt ecosystems and cause harm to plants, animals and humans.
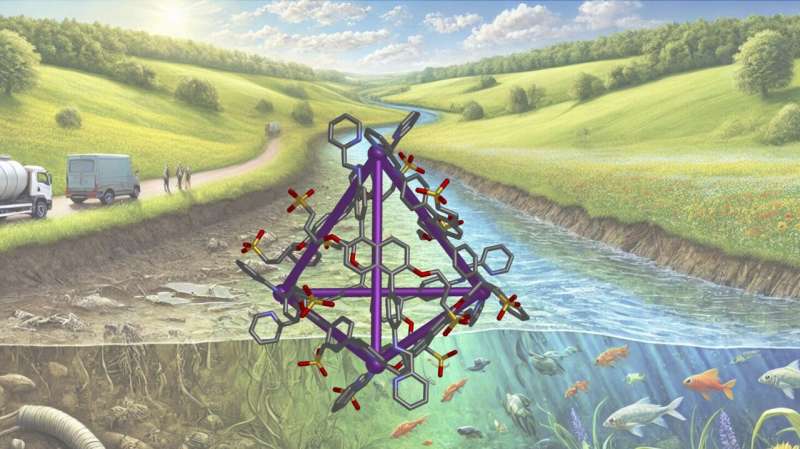
The research, published in the journal Cell Reports Physical Science, describes a new method using a molecular structure called a metal-organic cage (MOC). These tiny cages act like traps designed to catch and hold harmful molecules commonly found in our water supplies.
While MOCs have been studied before for gas and chemical capture, they are most commonly studied in chemical solvents where their performance differs significantly from that observed in water. Being able to demonstrate capture of established wastewater pollutants in water is thus a step towards the application of these cages for real-world applications.
Jack Wright, a Researcher at The University of Manchester, who completed the research as part of his Ph.D., said, “Being able to use MOCs in water is a really exciting development. We know how valuable MOCs are for capturing unwanted substances, but until now researchers have not been able to apply them to real-world water systems.
“Many harmful chemicals are difficult to remove from water, and with water pollution becoming a global crisis, this new MOC technology could provide a valuable tool to help clean up water systems and prevent pollutants from entering our ecosystem, particularly in rivers and lakes near urban or industrial areas where wastewater discharge is most common.”
The cages are made up of metal ions connected by organic molecules, forming a hollow pyramid-like structure. These hollow spaces at the center of these structures are where the MOCs trap specific molecules, like pollutants or gases.
The new structure incorporates chemical groups called sulfonates to make it compatible with water, allowing it to function in real-world water systems, like rivers or wastewater.
It uses a natural effect called hydrophobic binding, where contaminant molecules preferentially “stick” to the inside of the cage rather than staying in the water. This allows the material to selectively capture and hold pollutants, even in challenging water environments.
Dr. Imogen Riddell, Ph.D. supervisor and researcher at The University of Manchester, said, “One of the real strengths of this method is its flexibility. The approach we have developed could be used to design other water-soluble MOCs with different sizes or properties. This opens the door to many future applications, including cleaning up different kinds of pollutants, development of green catalysts or even development of drug delivery strategies .”
Now, the researchers will look to further expand the water-soluble cages, to enable capture of more and different contaminants, and are working towards the development of robust routes to recycling the cages to support their development as sustainable water purification aids.
More information:
Jack D. Wright et al, Encapsulation of Hydrophobic Pollutants within a Large Water-Soluble [Fe4L6]4- Cage, Cell Reports Physical Science (2025). DOI: 10.1016/j.xcrp.2025.102404
Provided by
University of Manchester
Citation:
Scientists create ‘molecular trap’ to remove pollutants from water (2025, January 28)
retrieved 28 January 2025
from https://phys.org/news/2025-01-scientists-molecular-pollutants.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.