Oxygen can destroy hydrogen-producing enzymes. Researchers from Bochum and Osaka have discovered how an extraordinary protein survives in the presence of oxygen.
[FeFe]-hydrogenases are the most efficient hydrogen (H2)-producing biocatalysts. However, most of their active centers, where H2 is produced, are destroyed by oxygen (O2), making it very difficult to used them in large-scale H2 production. An exception, [FeFe]-hydrogenase CbA5H, from the bacterium Clostridium beijerinckii, survives in the presence of oxygen.
An international collaboration led by the Photobiotechnology group at Ruhr University Bochum, Germany, and the protein crystallography group in Osaka University, Japan, headed by Professor Thomas Happe and Professor Genji Kurisu respectively, has revealed the molecular mechanism behind this in an article published in the Proceedings of the National Academy of Sciences.
CryoEM provides a complete picture of CbA5H
Three years ago, a partial structure of CbA5H solved in the presence of O2 showed that the active center is shielded by a nearby sulfur-containing group, which allowed us to only make a hypothesis about its O2-protection mechanism. However, this does not reveal how it produces hydrogen.
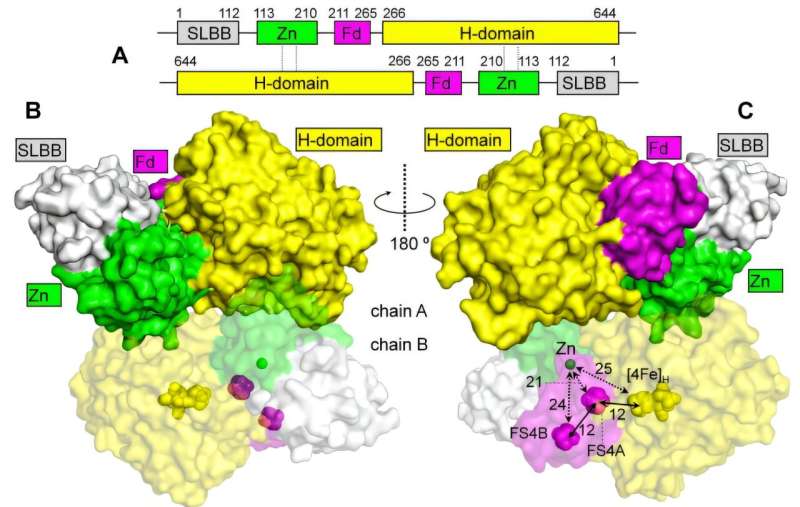
By collaborating with the Japanese group, the Bochum-based researchers have now obtained a complete structure of CbA5H under anoxic conditions using cryoEM. The new structure shows the shielding sulfur-containing group is detached to the active center, therefore representing the hydrogen producing state of CbA5H.
“Comparing both structures obtained under anoxic and aerobic conditions respectively allows us to conclude its working and O2 protection principles,” says Jifu Duan.
Zn2+-mediated dimerization increases the stability of CbA5H
In addition to acquiring an understanding of the workings of CbA5H and its O2 protection principles, the complete structure also provides important information in another aspect: CbA5H forms a homodimer (i.e., two CbA5H molecules are assembled together to form the minimal functional unit) and the homodimer is retained by a Zn2+-binding motif.
It is further demonstrated that the Zn2+-dependent homodimer is significantly more stable than the monomeric form when the Zn2+ was removed by a genetic modification.
“Taken all together, the study provides very good understandings in working mechanism, O2-protection strategy and dimerization-dependent stability, which would help in discovering new O2-resistant [FeFe]-hydrogenases,” concludes Happe.
More information:
Jifu Duan et al, Structural determinants of oxygen resistance and Zn2+-mediated stability of the [FeFe]-hydrogenase from Clostridium beijerinckii, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2416233122
Provided by
Ruhr-Universitaet-Bochum
Citation:
How nature optimizes hydrogen-producing biocatalysts (2025, January 15)
retrieved 15 January 2025
from https://phys.org/news/2025-01-nature-optimizes-hydrogen-biocatalysts.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.