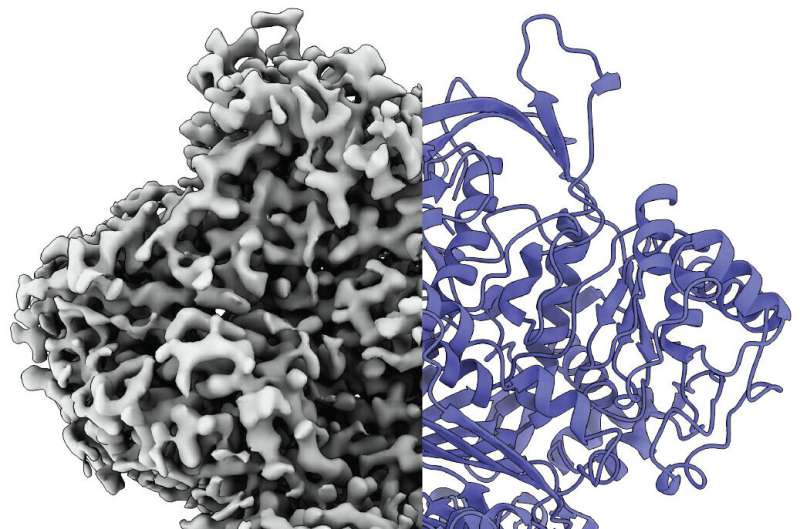
The protein “MIPS” changes its internal structure when it becomes active. Its disordered active center becomes a defined structure with special functions. The protein plays a key role in the production of inositol, which is also known as vitamin B8, and fulfills important tasks in the body.
Researchers at Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center in Greece have succeeded for the first time in observing the protein as it re-structures. As the team reports in the Proceedings of the National Academy of Sciences, this process appears to occur in many similar proteins.
Proteins control all vital processes in every organism, for example growth and metabolism. A basic principle of protein research is that the structure of each protein determines its function. If the structure is impaired at just one specific point, the protein can no longer fulfill its function. In the human body, this can lead, for example, to serious diseases.
However, there are numerous proteins that do not have a fixed structure, either in whole or in part. Not only are they notoriously difficult to analyze, their structure also changes depending on their environment.
“Proteins are often isolated from samples before they are analyzed. However, this does not allow us to see how they behave in their natural environment. We have developed a method that allows us to study proteins under almost native conditions,” explains Professor Panagiotis Kastritis, a biochemist at MLU.
His team analyzed samples of the fungus Thermochaetoides thermophila, which is used as a model organism in research. The work centered on the protein myo-inositol-1-phosphate synthase (MIPS), which is vital for the production of inositol. The substance is also known as vitamin B8 and is required for many important processes. However, since the body produces it, it is not considered a true vitamin.
“MIPS is part of a longer metabolic pathway that leads to the production of inositol,” explains Toni Träger from MLU. He had already investigated the protein as part of his master’s thesis and is now a member of Kastritis’s research group.
The scientists used cryo-electron microscopy to observe the protein at work. They discovered that it exists in at least three states: a disordered state, an ordered state and a type of third intermediate state.
“So far, we have not figured out definitely why this third state is needed. Perhaps it helps with the absorption of water, which facilitates subsequent reactions. Or perhaps something completely different is happening,” says Kastritis.
In a further step, the researchers investigated whether proteins related to MIPS exhibit similar behavior. MIPS is part of a special class of proteins known as isomerases. The team analyzed data on the structure of more than 340 other isomerases. And indeed, the researchers found clear indications of similar behavior.
The findings are not only of interest to basic research. “Greater knowledge of metabolic pathways and the proteins involved may open up new therapeutic approaches. Our work provides an important first step,” concludes Kastritis.
More information:
Toni K. Träger et al, Disorder-to-order active site capping regulates the rate-limiting step of the inositol pathway, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2400912121
Provided by
Martin Luther University Halle-Wittenberg
Citation:
From chaos to order: Proteins can re-structure themselves to create important substances (2024, September 23)
retrieved 24 September 2024
from https://phys.org/news/2024-09-chaos-proteins-important-substances.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.