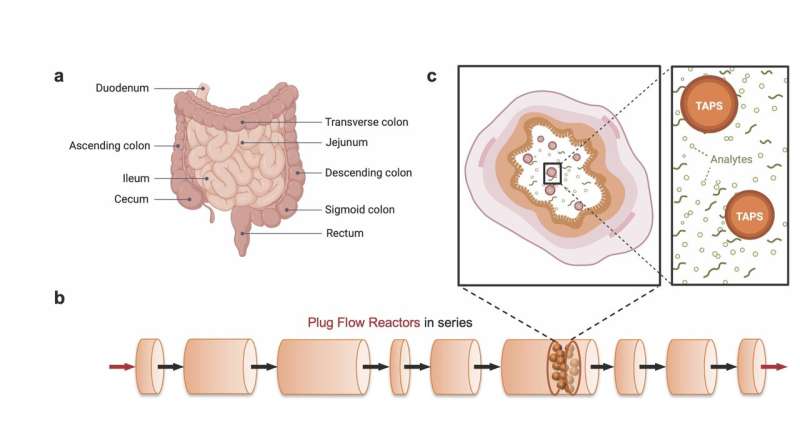
When synthesizing chemicals, stationary sensors can collect and communicate detailed data from within a reactor system. Physically installed sensors reach their limitations when it comes to mapping concentrations within a fluid flowing through hard-to-reach areas—particularly within long, narrow tubes.
While sensors can be placed on the reactor’s perimeter in an industrial setting, suspending sensors in the center of a pipe would disrupt flow. In a medical application, such as mapping chemical concentrations within the intestines to pinpoint internal bleeding, implanted sensors become impractical.
A new framework optimizes the use of time-aware particulate sensors (TAPS)—a tiny sensor that travels through the system and remembers when it encounters a target chemical—to map these uncharted areas.
Simulations demonstrate that when released in thousands, the sensors can collectively map out the entire concentration profile of these previously inaccessible systems. The University of Michigan study is published in the AIChE Journal.
“We show here that even with basic functions for each sensor, with strength in numbers, they achieve something together that is otherwise very hard to do,” said Albert Liu, an assistant professor of chemical engineering, macromolecular science and engineering and materials science and engineering at U-M and corresponding author of the study.
At only about 100 micrometers—about the width of a human hair—these particle-sized sensors are small enough to suspend in a fluid without significantly disrupting the flow pattern and large enough to be retrieved by filtration for analysis.
Sensor size and simplicity also help cut costs compared to traditional integrated circuit-based sensors, which are essentially shrunken computers. Millions of these sensors can be manufactured on a single silicon wafer—a disc about 12 inches or less in diameter.
The sensors keep track of time using an array of memristors—electrical components that store information using electrical resistance. Memristors, when arranged in a parallel circuit, can act as an analog clock. After the timer starts, each memristor in the ladder is switched off one at a time at a known rate. The clock ticks until a target chemical flips a switch in the sensor, shutting off the timer.
Once retrieved, the number of memristors switched off indicates how much time has passed since the sensor interacted with the chemical.
“It is a simple mechanism to turn temporal information into spatial information,” said Liu.
While memristors were the subject of this study, the system design is broad enough to encompass any time-sensitive detection method.
Models found that sensor performance was strongly impacted by factors like the type of chemical, concentration level, system size and length of time to pass through the system.
“Our simulation clearly reflected that sensors need to be tailored to their environment. There’s a kind of fingerprint to each system that changes the optimal number of sensors and the sensing material used to detect the target chemical,” said Matthew Manion, a doctoral student of chemical engineering at U-M and first author of the study.
To handle the differences between systems, the researchers developed an optimization scheme to help engineers design the best sensors for each unique system.
As the technology continues to develop, the research team hopes to add more spatial resolution to understand chemical concentrations in three dimensions. Currently, the method offers time resolution of fluid moving through a pipe—generating a concentration map of the chemicals inside the pipe.
The framework of deploying thousands of sensors ensures small enhancements on the sensor level will improve chemical concentration mapping by leaps and bounds.
Collectively, the sensors perform emergent functions—essentially, the system is much more capable than the sum of its parts.
The researchers could further harness the sensors’ collective emergent properties by enabling particles to communicate with each other.
“In this study, individual particles do not yet communicate with each other. A lot of emergent behavior relies upon particle to particle communication. When you have that, nonlinear scaling of such distributed particle-based systems truly comes alive,” said Liu.
More information:
Matthew Lee Manion et al, Temporally resolved concentration profiling via computationally limited, distributed sensor nodes, AIChE Journal (2024). DOI: 10.1002/aic.18691
Provided by
University of Michigan College of Engineering
Citation:
Thousands of tiny, time-aware sensors can collectively map chemical concentrations within narrow tubes (2025, February 3)
retrieved 3 February 2025
from https://phys.org/news/2025-02-thousands-tiny-aware-sensors-chemical.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.