In living organisms, protein molecules with different chemical structures called proteoforms, are produced from a single gene to perform a variety of physiological protein functions. It has been known for quite some time that humans have approximately 22,000 genes, but the total number of human proteoforms is still unknown.
Currently, liquid chromatography-mass spectrometry (LC-MS), a highly sensitive method for measuring biomolecules, is the main method used to analyze proteoforms. Whereas the traditional approach of analyzing proteoforms requires breaking them down into small peptides, a newer approach of analyzing intact proteoforms by LC-MS is called top-down proteomics.
Along the lines of the Human Genome Project that mapped all of our genes, there have been attempts to build an atlas of human proteoforms using top-down proteomics. However, the proteoform components extracted from biological samples are complex, and LC-MS alone cannot comprehensively detect all proteoforms.
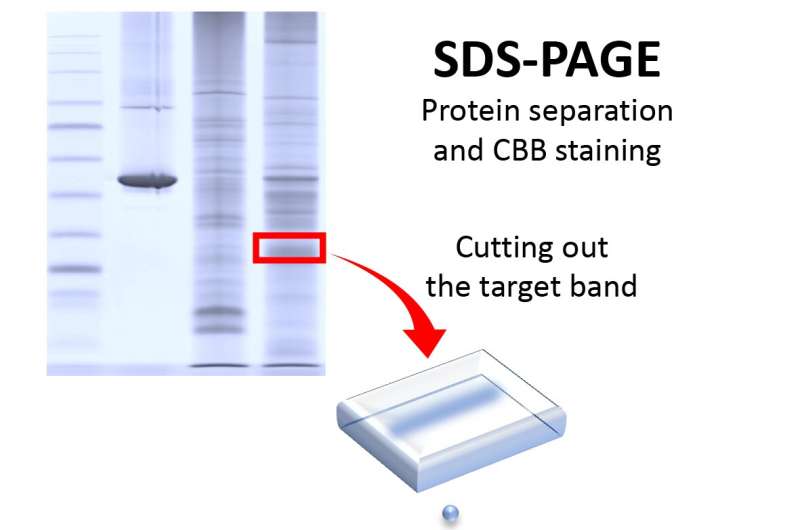
In 2020, the Takemori group at Ehime University originally developed PEPPI-MS, an innovative method for high-resolution fractionation of proteoforms using the inexpensive and simple SDS- PAGE system. Sample fractionation using PEPPI-MS has been successful in significantly increasing the number of proteoforms detectable by LC-MS, and its performance has led to the adoption of PEPPI in sample preparations, called “pre”-fractionation, in many top-down proteomics studies.
In 2022, in collaboration with Prof. Andreas Tholey’s group at the University of Kiel (Christian-Albrechts-Universität zu Kiel), Germany, an ultra-sensitive proteoform measurement system was built that combines PEPPI-MS fractionation with FAIMS ion mobility mass spectrometry that can separate proteoforms in the gas phase.
Using this system, in 2023 they achieved detailed analysis of human cultured cells via top-down proteomics and established a methodology for a new middle-down proteomics, in which the Glu-C digestion products of proteoforms are analyzed.
PEPPI-MS fractionation does not require any special equipment and can be performed with standard biochemical laboratory equipment. Its ease and accessibility has led to its becoming a standard method for sample fractionation in deep top-down proteomics.
To further promote proteoform analysis, the Takemori group established experimental protocols for high-resolution proteoform fractionation applying PEPPI-MS and top-down/middle-down proteomics using the FAIMS-LC-MS system and recently published a streamlined protocol combining their work from 2020 to 2024 in Nature Protocols.
The complete set of PEPPI-MS protocols enables high-resolution fractionation of trace biological samples and large-scale proteoform analysis by LC-MS and is expected to contribute to the construction of proteoform atlases for various living species and the development of disease diagnostic methods based on precise proteoform information.
More information:
Ayako Takemori et al, PEPPI-MS: gel-based sample pre-fractionation for deep top-down and middle-down proteomics, Nature Protocols (2025). DOI: 10.1038/s41596-024-01100-0
Provided by
Ehime University
Citation:
Streamlined protocol reveals chemical structure of cellular proteins on a large scale (2025, January 27)
retrieved 27 January 2025
from https://phys.org/news/2025-01-protocol-reveals-chemical-cellular-proteins.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.