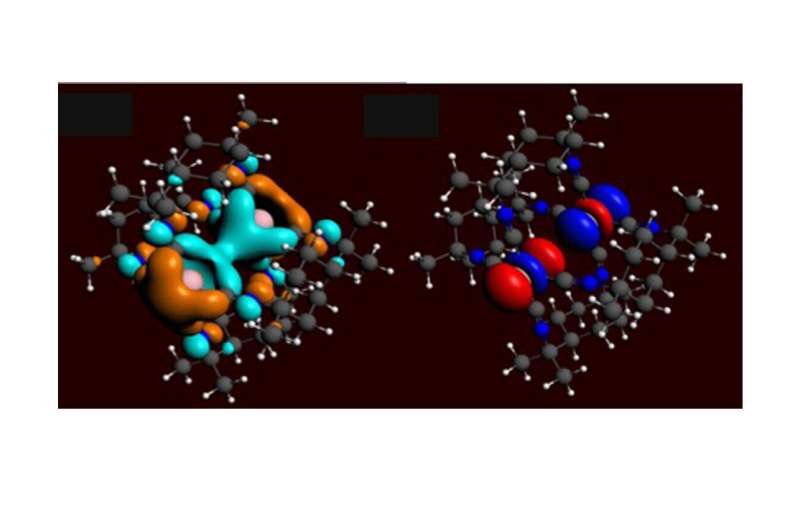
Attractive metal-metal bonding occurs in a variety of molecules made of metallic atoms such as Iridium (Ir). These molecules can have different spatial arrangements, called isomers. Current theoretical and experimental methods have shown the existence of two isomers for Ir-Ir molecular systems. However, researchers have not been able to predict the proportions of these two isomers or how they interact.
Recent research combines ultrafast experimental measurements and numerical simulations to tease out key information about the two isomers in Ir-Ir complexes. The researchers also used the experimental data to assess which numerical methods best fit reality.
The work is published in the Journal of the American Chemical Society.
Metal dimer complexes with d8 square planar metallic centers provide a class of configurable molecules that may have applications in sensing and catalysis due to the relationship between the metal-metal distance and the optical and reactive properties of the molecule. Understanding and predicting the equilibrium spatial geometries of these molecules is essential for controlling their chemical properties. The Ir-Ir metallic dimer complexes studied here have two possible spatial geometries (also referred to as isomers) in equilibrium, referred to as the short and long configurations due to the distance between Ir atoms in each configuration.
In this study, the authors used an excitation process called optical hole burning to selectively deplete the population of the long isomer from the ground state. This creates an imbalance that the system seeks to rectify by re-equilibrating. The researchers tracked re-equilibration using ultrafast X-ray solution scattering at the Linac Coherent Light Source (LCLS).
This DOE Office of Science light source user facility produces ultrashort pulses of high energy X-rays that provide the necessary time and spatial resolution to follow the dynamics of these systems. This data is used to extract the ground state proportion of the isomers and the transition rate between them. The researchers then used this experimental data in tandem with numerical solutions to answer long-standing questions about which DFT approximations are appropriate to accurately predict experimental outcomes.
This joint experimental-theoretical work reveals the forces driving ultrafast equilibration in metal-metal bonding. This is important to researchers designing configurable molecules for use in sensing and catalysis. The research compared many different density functional theory (DFT) approximations with experimental data. DFT is a way to model quantum mechanical systems. These comparisons help to identify the best way to create accurate numerical models of metal-metal bonding. The results point toward a systematic way to establish which isomers are present in the ground state for a variety of metal-metal complexes.
More information:
Natalia E. Powers-Riggs et al, Characterization of Deformational Isomerization Potential and Interconversion Dynamics with Ultrafast X-ray Solution Scattering, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c00817
Provided by
US Department of Energy
Citation:
Advanced techniques paint a more accurate picture of molecular geometry in metal complexes (2025, January 13)
retrieved 13 January 2025
from https://phys.org/news/2025-01-advanced-techniques-accurate-picture-molecular.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.