Microorganisms have long used hydrogen as an energy source. To do this, they rely on hydrogenases that contain metals in their catalytic center. In order to use these biocatalysts for hydrogen conversion, researchers are working to understand the catalysis process.
A team from three Max Planck Institutes (MPI), the Center for Biostructural Imaging of Neurodegeneration (BIN) at the University Medical Center Göttingen (UMG), the University of Kiel, and the FACCTs GmbH used a chemical peculiarity of hydrogen to amplify the signals of magnetic resonance spectroscopy. In this way, the scientists were able to visualize previously unknown intermediate steps in the conversion of hydrogen. The study is published in Nature Catalysis.
As a substitute for fossil fuels, energy source, or catalyst in chemical processes—hydrogen is considered a good candidate for a sustainable energy economy. On Earth, the element occurs mainly in bound form, in water, as hydrogen gas, or in fossil raw materials such as natural gas and crude oil. To obtain hydrogen in its pure form, it must be split from the chemical compound using energy.
The most common method of producing hydrogen today is the steam methane reforming of natural gas. However, this also produces climate-damaging carbon dioxide (CO₂). In the catalytic production of hydrogen from water, electrodes made of the precious metal platinum have mostly been used up to now. This makes hydrogen production by means of catalysis comparatively expensive.
Many microorganisms are a step ahead of these production processes. To split off hydrogen to generate energy, they use three different types of hydrogenases that function without precious metals and do not release CO2: [NiFe] hydrogenases from archaea and bacteria, [FeFe] hydrogenases from bacteria, some algae, and some anaerobic archaea, as well as [Fe] hydrogenases found only in archaea.
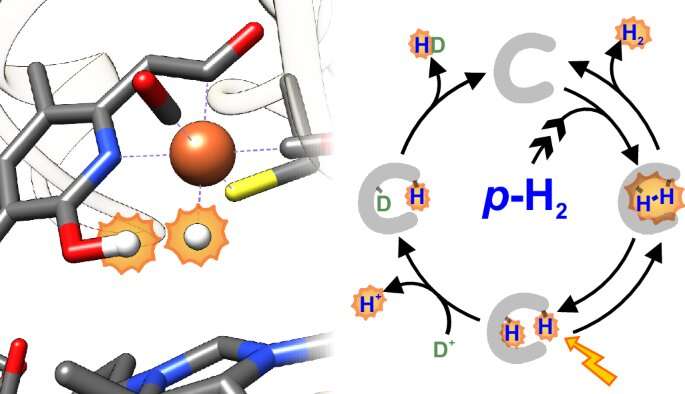
The latter play a key role in methanogenesis, in which CO2 is reduced to methane (CH4). The homodimeric [Fe] hydrogenase contains one redox-inactive iron (Fe) per subunit, which is bound to a guanylylpyridinol cofactor.
While intermediates in the catalytic cycle of [NiFe] hydrogenases and [FeFe] hydrogenases have already been well studied, the catalytic intermediates of [Fe] hydrogenases were not observable—until now. A research team has now succeeded in detecting the intermediates in the [Fe]-hydrogenases catalysis cycle for the first time.
The team was led by Stefan Glöggler (Max Planck Institute for Multidisciplinary Sciences (MPI-NAT) and the Center for Biostructural Imaging of Neurodegeneration (BIN) at the University Medical Center Göttingen (UMG), Lukas Kaltschnee (MPI-NAT and BIN at UMG, currently at the TU Darmstadt), Christian Griesinger (MPI-NAT), and Seigo Shima (MPI for Terrestrial Microbiology), and included colleagues from the MPI für Kohlenforschung, Kiel University, and the FAccTs GmbH.
The researchers made use of the fact that hydrogen occurs as so-called parahydrogen and orthohydrogen, depending on its nuclear spin. The researchers showed that nuclear magnetic resonance spectroscopy results in signal amplification when the [Fe] hydrogenase reacts with parahydrogen. This so-called parahydrogen-induced polarization (PHIP) made it possible to identify the reaction intermediates and visualize how the [Fe] hydrogenase binds hydrogen during catalysis.
The scientists’ data indicate that a hydride is formed at the iron center during catalysis. The new method also made it possible to study the binding kinetics. Due to its high sensitivity, PHIP is particularly promising for application to living cells and for investigating hydrogen metabolism in vivo. The results could help to develop (bio)catalysts for hydrogen conversion with higher productivity in the future.
More information:
Lukas Kaltschnee et al, Parahydrogen-enhanced magnetic resonance identification of intermediates in the active [Fe]-hydrogenase catalysis, Nature Catalysis (2024). DOI: 10.1038/s41929-024-01262-w. www.nature.com/articles/s41929-024-01262-w
Provided by
Max Planck Society
Citation:
Hydrogen’s dual nature helps reveal hidden catalytic processes (2024, December 13)
retrieved 14 December 2024
from https://phys.org/news/2024-12-hydrogen-dual-nature-reveal-hidden.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.