A team of researchers from the Hebrew University of Jerusalem, the Weizmann Institute of Science, and the University of Tokyo has made a significant breakthrough in the fight against cancer by developing a highly selective inhibitor for an enzyme called Matrix Metallopeptidase 7 (MMP7). This enzyme plays a crucial role in cancer progression, especially in helping tumors invade surrounding tissues and spread to other parts of the body (metastasis). The findings are published in the journal Angewandte Chemie International Edition.
MMP7 has long been recognized as an attractive target for cancer therapies, but creating drugs that specifically block it has proven challenging due to the structural similarities it shares with other related enzymes in the matrix metalloproteinase family. These enzymes have overlapping functions, which makes it difficult to design a drug that targets only MMP7 without affecting others.
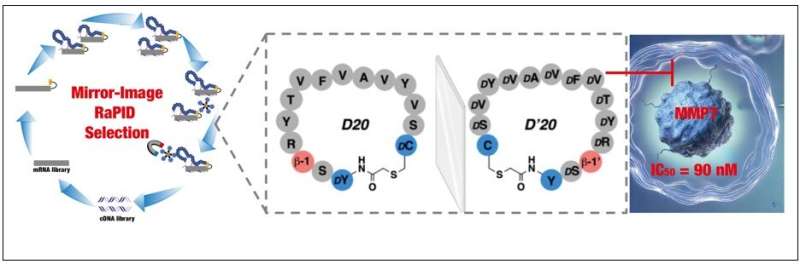
Led by Professor Norman Metanis and Ph.D. student Hiba Ghareeb from the Hebrew University, in collaboration with Professor Irit Sagi from the Weizmann Institute of Science and Professor Hiroaki Suga from the University of Tokyo, the study utilized a pioneering approach called Mirror-Image Random Nonstandard Peptide Integrated Discovery (MI-RaPID). This advanced technology enabled the research team to identify a new class of molecules—known as macrocyclic peptides—that bind specifically to MMP7, inhibiting its activity without interfering with similar enzymes.
One of the standout peptides discovered in this study, named D’20, was designed in a unique mirror-image form. This peptide is made up of twelve specially modified building blocks called D-amino acids, along with other structural elements, making it both stable and highly specific.
In laboratory tests, D’20 demonstrated an impressive ability to block MMP7 activity with a high degree of precision, without affecting other enzymes that have similar functions. Furthermore, D’20 was shown to stop the movement of pancreatic cancer cells, a critical step in halting metastasis, while leaving the normal growth of the cells unaffected.
The stability of D’20 was also remarkable—it retained its structure and function in human blood and in conditions that simulate the digestive system, suggesting it has great potential for further development as a drug. This level of stability is key in ensuring that the peptide can work effectively in the human body over time.
This research not only offers new hope for targeting MMP7 in cancer therapy but also showcases the power of mirror-image random peptide discovery technology in developing new treatments. By creating highly specific and stable peptides, the team has opened up exciting possibilities for treating aggressive cancers, like pancreatic cancer, and improving the outlook for patients facing these difficult diseases.
More information:
Norman Metanis et al, Mirror‐Image Random Nonstandard Peptide Integrated Discovery (MI‐RaPID) Technology Yields Highly Stable and Selective Macrocyclic Peptide Inhibitors for Matrix Metallopeptidase 7, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202414256
Provided by
Hebrew University of Jerusalem
Citation:
Highly-stabilized and selective inhibitor for cancer-causing enzyme developed (2024, October 15)
retrieved 16 October 2024
from https://phys.org/news/2024-10-highly-stabilized-inhibitor-cancer-enzyme.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.