Using ammonia is regarded as a promising method of transporting hydrogen. However, an efficient process is also needed to convert it back into hydrogen and nitrogen.
An international research team has gained new insights into the mode of operation of an iron catalyst that can be used to split ammonia into nitrogen and hydrogen. Hydrogen is converted into ammonia to make the energy carrier easier to transport. This means that catalysts are also needed that can subsequently break ammonia down into its starting materials again.
A team from the German Ruhr University Bochum, the Max Planck Institute for Chemical Energy Conversion (MPI CEC) in Mülheim an der Ruhr, Technische Universität Berlin and the Italian Institute of Technology in Genoa describes how the iron catalyst enables this reaction in detail in an article published in ACS Catalysis.
How to make hydrogen transportable
Green hydrogen is regarded as a promising energy carrier. It can be produced by splitting water using wind or solar energy. However, in many cases the locations where hydrogen is needed don’t provide the right conditions for water electrolysis.
Hydrogen must be liquefied for transportation, which is only possible at extremely low temperatures. Converting hydrogen into ammonia, which can be liquefied at much higher temperatures, is therefore considered an attractive alternative.
“What’s more, the chemical industry already has an established infrastructure for ammonia handling,” says Professor Martin Muhler, Head of the Laboratory of Industrial Chemistry in Bochum and Max Planck Fellow at the MPI CEC.
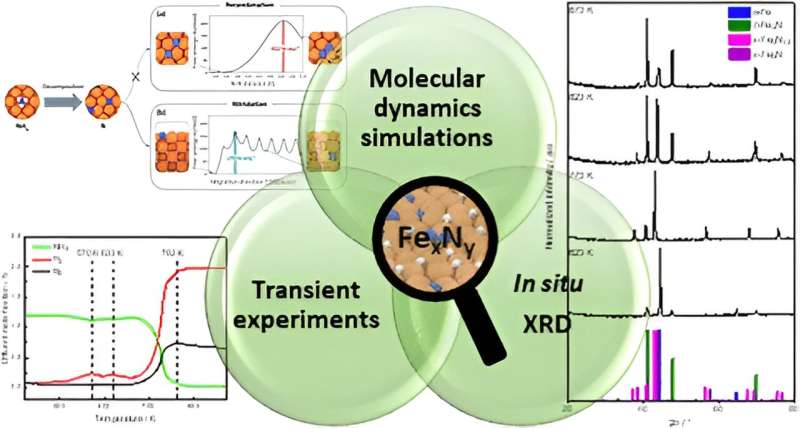
Efficient catalysts are needed to break down ammonia (NH3) back into its starting compounds nitrogen (N2) and hydrogen (H2). The problem is that conventional iron catalysts generally facilitate an undesirable reaction to form iron nitride instead of nitrogen.
In the current study, the researchers have shown exactly how this side reaction occurs. They tested ammonia decomposition using a catalyst of the latest generation provided by Clariant.

The team consisting of Dr. Maximilian Purcel, Astrid Müller and Professor Martin Muhler from Ruhr University Bochum and MPI CEC carried out the relevant experiments. The findings were refined using complex molecular dynamics simulations, supported by machine learning, which were conducted by the Italian partner institute. The team from Technische Universität Berlin successfully identified the iron nitrides formed under reaction conditions using X-ray diffraction and tracked their transformations.
“Our findings can be used to develop more efficient catalysts for splitting ammonia in the future,” concludes Martin Muhler. “Ammonia synthesis and decomposition has a long track record,” he adds. “We cite scientific publications from the past 100 years.” These include the work of Martin Muhler’s doctoral supervisor Gerhard Ertl, who was awarded the Nobel Prize for his research in 2007.
More information:
M. Purcel et al, Iron Nitride Formation and Decomposition during Ammonia Decomposition over a Wustite-Based Bulk Iron Catalyst, ACS Catalysis (2024). DOI: 10.1021/acscatal.4c04415
Provided by
Ruhr-Universitaet-Bochum
Citation:
Iron catalyst study provides insights into ammonia decomposition (2024, October 8)
retrieved 8 October 2024
from https://phys.org/news/2024-10-iron-catalyst-insights-ammonia-decomposition.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.