Combining two techniques, analytical chemists at the Department of Energy’s Oak Ridge National Laboratory have become the first to detect fluorine and different isotopes of uranium in a single particle at the same time. Because fluorine is essential for converting uranium into a form suitable for enrichment, spotting both elements together may help inspectors of the International Atomic Energy Agency, or IAEA, determine the intended use of a nuclear material.
The findings, published in the Journal of the American Chemical Society, push the limit of how fast single particles can be characterized in terms of their chemical, elemental and isotopic compositions. Critical for understanding chemical processes and dating materials, isotopes are different forms of a chemical element having the same number of protons but a different number of neutrons.
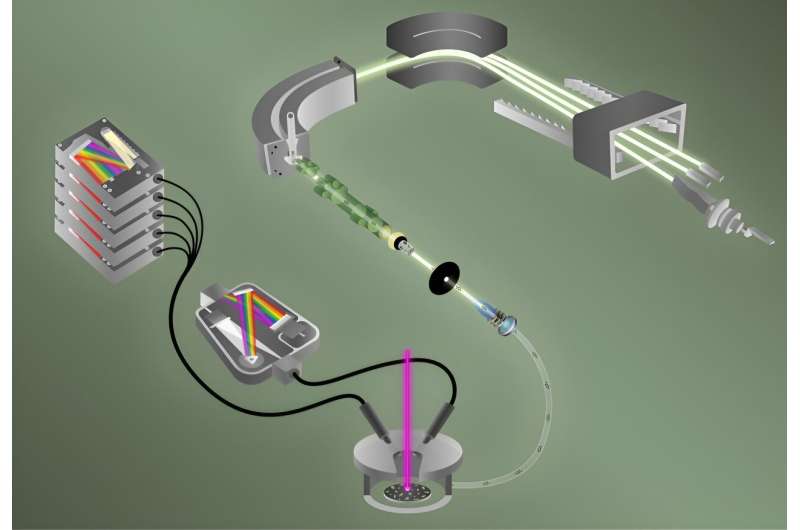
“Determining isotopic ratios on single particles takes a lot of time,” said ORNL’s Benjamin Manard, who led the study. “Rapid particle analysis for fluorine and uranium isotopic determination is what we’ve enabled.” His team combined two techniques to analyze 40 particles—each about the size of a red blood cell—in less than five minutes.
The first technique is laser-induced breakdown spectroscopy, or LIBS. It quickly spots the element fluorine with great sensitivity.
“LIBS vaporizes a sample, like a uranyl fluoride particle, breaking it down and forming a plasma, or cloud of excited ions. As the plasma cools, light is emitted,” said ORNL’s Hunter Andrews, the study’s LIBS task lead. Spectroscopy then measures the light to characterize elements in the plasma.
“It’s like fireworks,” Manard said. “Different elements emit different colors, or wavelengths.”
Simultaneously, helium gas sweeps atoms of the plasma into a mass spectrometer, where isotopes of uranium are characterized via the second technique, called laser ablation multicollector inductively coupled plasma mass spectrometry, or ICP-MS. Inductively coupled means radio-frequency energy heats the plasma. It reaches 8,000 kelvin—hotter than the surface of the sun.
“LIBS tells us if and how much fluorine is in the particle, while ICP-MS tells us all of the uranium isotopes present,” Manard said. “This integrated equipment is a one-stop shop to measure fluorine as well as uranium isotopes at the same time.”
Uranyl fluoride, a molecule containing uranium, oxygen and fluorine, indicates the occurrence of certain nuclear processes. “Detection of uranium and fluorine in the same particle is meaningful from a nuclear nonproliferation point of view,” said ORNL co-author Brian Ticknor. “Being able to determine how much fluorine versus uranium is present can give you additional information about where that particle came from, what processes produced that particle and how long ago those processes occurred.”
He added, “Even though these tools are developed for national security purposes, they could have applications to varied fields, including next-generation battery manufacturing, fuels for advanced nuclear reactor design and environmental science revealing the transport and fate of microplastics.”
Why had no one put these two pieces of equipment together before?
“The ICP-MS needs a positive charge to make our measurement,” Ticknor said. “Uranium, plutonium, most metals, a lot of the periodic table, are happy to be positive ions. Part of the reason that we need to do this simultaneous technique is because elements like fluorine are not amenable to the ICP-MS that we do for uranium. It has to do with the electronegativity of fluorine. With ICP, the goal is to form positive ions to inject into the mass spectrometer. Fluorine very, very strongly wants to have a negative charge.”
Manard explained further, “That’s why we needed to do the LIBS for the fluorine piece of this and then the mass spectrometry for the uranium isotopic determination. Many people have done LIBS and ICP-MS separately, but no one had done both in a multicollector-based fashion.”
Manard conceptualized experiments for a multidisciplinary team at ORNL’s Ultra-trace Forensic Science Center. The center’s advanced characterization of nuclear materials provides a better understanding of novel fuel cycle processes.
Ticknor, Cole Hexel and Paula Cable-Dunlap, all of ORNL, served as advisors to the project. Andrews performed LIBS experiments. Daniel Dunlap and Alex Zirakparvar, both of ORNL, readied the ICP-MS instrument to make high-precision isotope ratio measurements. ORNL’s Tyler Spano prepared samples and benchmarked them using Raman spectroscopy, a traditional method to measure chemical composition that can take weeks.
Veronica Bradley, a former ORNL postdoctoral fellow, helped set up maps for laser ablation. C. Derrick Quarles Jr. of Elemental Scientific Inc, which manufactures the LIBS system, helped set up methods and tune equipment.
Manard and Ticknor envision applications for their technique beyond nuclear nonproliferation. In fact, when ORNL bought the LIBS instrument to target challenging elements such as fluorine, the research gained teeth—literally. Manard led experiments with researchers at Savannah State University, the University of Maine and ORNL using the new equipment to map fluorine distribution in shark teeth obtained from the Georgia Aquarium. The enamel of shark teeth is rich in fluorine and revealed environmental conditions, both current and prehistoric.
The innovative integration of the two techniques can reveal the origin and evolution of isotopes in diverse fields, including geology, biology, chemistry and nuclear materials.
“My research has recently centered around high-throughput particle analysis,” said Manard. “Due to improvements in laser ablation, we’ve been able to push the limits of how fast we can characterize single particles. We’re trying to push through thousands of particles in 24 hours with this technology. Speed is always in the back of my mind. “
Having used the joint techniques to differentiate uranium oxide from uranyl fluoride, the researchers are keen to dig deeper.
“What would be really cool and interesting is if we can distinguish other types of uranium compounds,” Manard said.
The team is also eager to extend the techniques to other challenging compounds relevant to nuclear processes, such as those containing chlorine, which is electronegative like fluorine.
“Chlorine is right below fluorine on the periodic table and has a lot of the same properties,” Ticknor said. “Uranium chloride has been hard to measure in the past, and it would potentially be amenable to this type of measurement.”
More information:
Benjamin T. Manard et al, Uranium Single Particle Analysis for Simultaneous Fluorine and Uranium Isotopic Determinations via Laser-Induced Breakdown Spectroscopy/Laser Ablation–Multicollector–Inductively Coupled Plasma–Mass Spectrometry, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c03965
Provided by
Oak Ridge National Laboratory
Citation:
Simultaneous detection of uranium isotopes and fluorine advances nuclear nonproliferation monitoring (2024, September 26)
retrieved 26 September 2024
from https://phys.org/news/2024-09-simultaneous-uranium-isotopes-fluorine-advances.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.