In just two neutron experiments, scientists discovered remarkable details about the function of an enzyme that can aid drug design for aggressive cancers.
The scientists, working at the Department of Energy’s Oak Ridge National Laboratory, used neutrons at the Spallation Neutron Source and the High Flux Isotope Reactor to identify exact atomic-scale chemistry in serine hydroxymethyltransferase, or SHMT, a metabolic enzyme necessary for cell division.
Cancer hijacks chemical reactions in the metabolic pathway that involves SHMT and other critical enzymes and turns the entire process into a runaway train, rapidly reproducing cancer cells. Designing an inhibitor to block the enzyme’s function, which falls early in the metabolic pathway, could derail cancer’s attempts to overtake it. The results were published in Chemical Science.
“I think neutrons will be highly sought after in future structure-based drug design,” said ORNL’s Victoria Drago, the lead author and a biochemist working in collaboration with Andrey Kovalevsky, a distinguished R&D scientist at ORNL, who uses neutron diffraction to illuminate protein structures.
“This paper is a good example of how quickly neutrons can produce information that has been the subject of debate for a very long time. Studies on SHMT function and its catalytic mechanism date back to the early 1980s.”
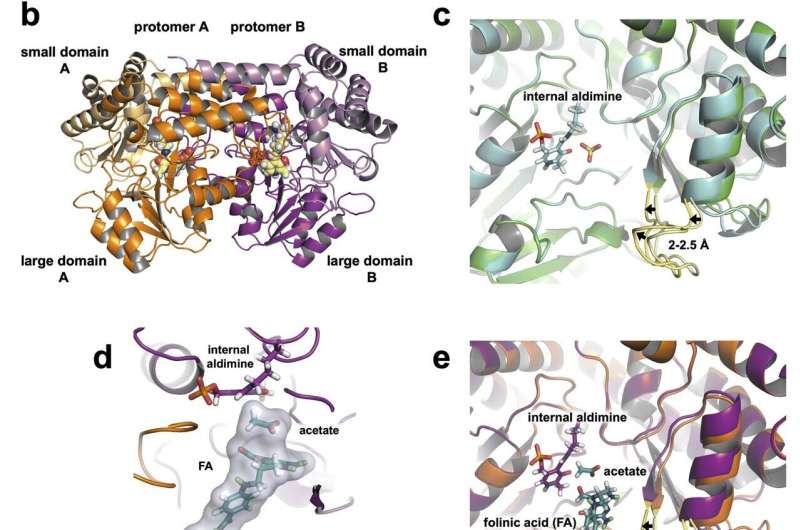
The exact catalytic mechanism and the roles of various amino acid residues in the enzyme’s active site have been debated for decades. In the current study, the researchers observed that just one amino acid residue, a glutamate, regulates chemical reactions for this enzyme.
“The neutron data clearly show that the glutamate, which is an acid, has the proton on it,” said co-author Robert Phillips, a professor of chemistry at the University of Georgia. “You might expect it to already have given up its proton. But because it’s able to carry that proton around, it can transfer it back and forth. So, it acts as an acid and a base.”
In a pathway known as one-carbon metabolism, this enzyme works inside a cell’s mitochondria, or energy producer. It converts the amino acid serine into another amino acid called glycine by transferring a carbon atom to tetrahydrofolate, a reduced form of folic acid. This reaction produces building blocks for the synthesis of nucleic acids, such as DNA and RNA, and other biological molecules critical to cell division. The glutamate controls this process.
In a prior experiment, the team combined two techniques, neutron and X-ray crystallography at physiologically relevant room temperature, to understand SHMT and to map its protein structure before its interaction with tetrahydrofolate. In the current experiment, the researchers captured the enzyme at the next step, establishing certainty about how the enzyme’s reaction mechanism actually works.
Painting the picture with neutrons
Neutrons see light elements, such as hydrogen, and X-rays see heavier elements, such as carbon, nitrogen and oxygen. Neutron diffraction at SNS and HFIR, in-house X-ray diffraction at ORNL and synchrotron X-ray diffraction at Argonne National Laboratory’s Advanced Photon Source provided insights the team needed to definitively characterize the enzyme’s chemical reaction.
“Neutrons allow us to see hydrogen atoms, and hydrogen drives chemistry,” Drago said. “Enzymes are about 50% made up of hydrogen atoms. In terms of electrostatics, hydrogen also carries a positive charge, which dictates the environment of the enzyme. Once you have a crystal that will diffract neutrons, you have everything you need. You see the positions where hydrogens are located and, equally as important, the positions lacking hydrogens. You get the whole picture.”
As shown in the animation, cancer cell mitochondria overproduce the SHMT enzyme, a tetramer constructed from four identical peptide chains, or protomers, shown in gray. SHMT functions by using pyridoxal-5′-phosphate, covalently bound to SHMT, and tetrahydrofolate, shown in gold and purple, respectively.
Tetrahydrofolate acts as a substrate that binds to the active sites of all four protomers. The hydrogen atoms, shown flashing in green, revealed the exact catalytic mechanism and the roles of various amino acid residues in the enzyme’s active sites. Once the enzyme releases tetrahydrofolate, an inhibitor, shown in blue, could be designed to block further chemical reactions at these sites, arresting the one-carbon metabolic pathway in cancer cells.
“The locations of the hydrogen atoms determine protonation states of specific chemical groups inside the enzyme’s active sites,” Kovalevsky said. “Thus, they provide information on the electric charge distribution, or electrostatics. This knowledge is crucial to designing small-molecule inhibitors that would bind to SHMT, replacing tetrahydrofolate and halting the enzyme function.”
Cells contain thousands of enzymes functioning as catalysts that speed up biochemical reactions needed for bodily functions—from breathing to producing hormones to nerve function. Enzymes also provide a place to tuck chemicals that target specific processes.
Other enzymes in the one-carbon metabolic pathway are already well-known targets for cancer drugs such as methotrexate and fluorouracil. However, SHMT comes earlier in this pathway, presenting an opportunity to stop cancer earlier.

But the difficulties with treating cancer relate in part to its stealthy attacks on metabolic processes. Unlike drug resistance in infectious diseases, if one path does not work well, cancer recalibrates other metabolic processes to overproduce cancer cells.
“Now that we know the atomic details for SHMT, we can inform the design of an inhibitor to target this specific protein as part of a combination therapy,” Kovalevsky said.
“If you compare it to treating infectious diseases, this is much more difficult because in cancer chemotherapy, you usually target your own proteins, which is why patients experience side effects. In infectious diseases, the proteins you target belong to the viruses or the bacteria. But with cancer, you have to kill your own cells. The idea here is to kill the cancer sooner and have less of an effect on the patient.”
Speeding the pace of discovery
The team used neutrons at the MaNDi instrument at SNS and the IMAGINE instrument at HFIR for its research. ORNL’s recent Proton Power Upgrade project added stronger beams for all the instruments at SNS. Stronger proton beams mean more neutrons. More neutrons mean shorter data collection times with smaller samples, speeding answers that help the scientists design smarter drugs to treat diseases.
“Discovery research is absolutely essential,” said William Nelson, director of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, who was not an author of either ORNL-led study. “We’re moving ever closer to the space where, with the help of AI, we will be able to sequence a gene in somebody’s cancer, predict what the protein structure would look like and make a drug to tuck in; it will work great, and we’ll do it in an hour and a half.
“But we’re not there yet. So, the more we know about the actual protein structure, chemical structure and the way things interact, the better we’re going to be able train AI models to predict things we don’t know right away.”
More information:
Victoria N. Drago et al, Universality of critical active site glutamate as an acid–base catalyst in serine hydroxymethyltransferase function, Chemical Science (2024). DOI: 10.1039/D4SC03187C
Provided by
Oak Ridge National Laboratory
Citation:
Neutron experiments settle 40-year debate on enzyme for drug design (2024, September 24)
retrieved 26 September 2024
from https://phys.org/news/2024-09-neutron-year-debate-enzyme-drug.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.