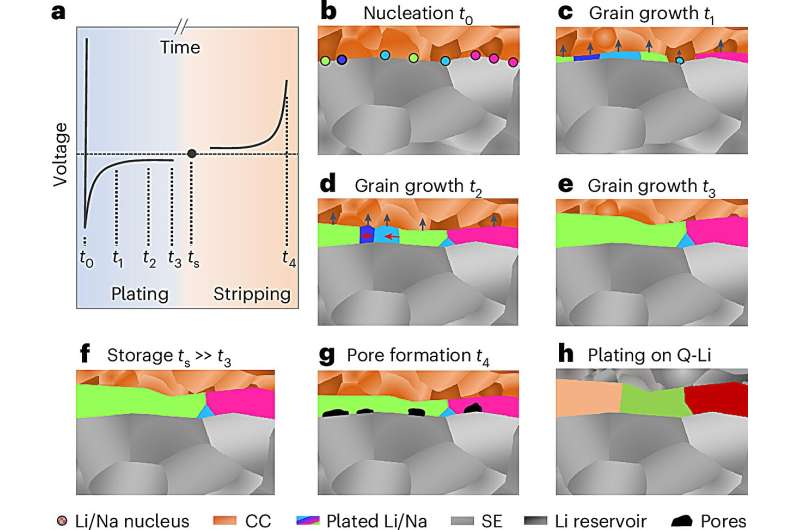
Lithium and sodium metal anodes play a crucial role in the further development of high-performance solid-state batteries. In order to favorably influence the electrochemical properties of these highly reactive alkali metals, understanding their microstructure is essential.
For the first time, a method developed at Justus Liebig University Giessen (JLU), in collaboration with an international research team from the U.S. and Canada, has succeeded in elucidating the microstructure of alkali metals deposited in a battery.
The elucidation of the microstructure of lithium or sodium opens up entirely new approaches to influencing the properties of the battery. The results have been published in Nature Materials.
The microstructure of metals—i.e., their internal structure on the scale of a few nanometers to several micrometers—is crucial in determining their electrochemical properties. For most metals that are technologically utilized today, this structure has been extensively studied, and in many cases, specific properties can be achieved through targeted control of the microstructure.
However, the situation has been different for the alkali metals lithium and sodium used in batteries. This is because these metals are chemically very reactive. Their surfaces are almost immediately covered with thick reaction layers in nearly all environments, making it impossible to determine their microstructure.
But now, researchers in materials science and chemistry from JLU, the University of California, Santa Barbara (U.S.), and the University of Waterloo (Canada) have demonstrated, for the first time, a method to determine the microstructure of both electrochemically deposited lithium and sodium metal.
For this purpose, the team from Giessen, led by Prof. Dr. Jürgen Janek from the Institute of Physical Chemistry at JLU, developed a sequence of preparation and analysis steps at very low temperatures and under inert gas conditions, culminating in the determination of the local metal structure using so-called electron backscatter diffraction.
With this method, the team was able to demonstrate how electrochemically grown metal layers of lithium and sodium, with thicknesses of up to 100 micrometers, are structured.
“The grain size of the produced layers was surprising to us, and the results provide important insights into the growth mechanism,” said Janek, who is also a member of the POLiS (Post Lithium Energy Storage) excellence cluster.
“The findings on sodium metal will also give a strong boost to the work in POLiS, whose mission includes the exploration of sodium batteries.”
The development of solid-state batteries is associated with the hope for particularly powerful, safe, and long-lasting electrochemical energy storage systems. The use of ceramic solid electrolytes could enable the application of lithium and sodium metal electrodes in high-performance batteries.
However, there are still challenges in using metal electrodes, especially due to the strong tendency of the metals to deform during electrochemical cycling. This affects both the charging and discharging processes.
During battery discharge, pores form in the metal, while during metal deposition in the charging step, microscopic metal structures, known as dendrites, often form, which can cause short circuits.
In the pursuit of more efficient solid-state batteries that can compete with conventional lithium-ion batteries, lithium (or sodium) metal should ideally only form during the first charging step, to avoid the handling difficulties associated with highly reactive alkali metal foils.
For about 10 years, the development of solid-state batteries has been driven by intensive research efforts worldwide, and the team from Giessen, led by Prof. Janek, is among the world’s leading research groups. Prof. Janek has successfully collaborated for years with the teams in Santa Barbara and Waterloo, who were significantly involved in this study.
“Imaging the microstructure of lithium and sodium was considered very challenging and had only rarely been reported—and even then, only from simple foil surfaces.
“Thanks to the meticulous preliminary work, the two lead authors of our study succeeded in cutting lithium and sodium electrodes, preparing them in cross-section, and imaging them using electron backscatter diffraction,” says Janek.
“This success was only possible through the consistent collaboration of various specialists at JLU and the excellent equipment of the Center for Materials Research. The collaboration with colleagues in Santa Barbara and Waterloo was also crucial for the proper material selection.”
More information:
Till Fuchs et al, Imaging the microstructure of lithium and sodium metal in anode-free solid-state batteries using electron backscatter diffraction, Nature Materials (2024). DOI: 10.1038/s41563-024-02006-8
Provided by
University of Giessen
Citation:
Electron backscatter diffraction elucidates the microstructure of alkali metals deposited in a battery (2024, September 25)
retrieved 25 September 2024
from https://phys.org/news/2024-09-electron-backscatter-diffraction-elucidates-microstructure.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.