In nature, photosynthesis powers plants and bacteria; within solar panels, photovoltaics transform light into electric energy. These processes are driven by electronic motion and imply charge transfer at the molecular level. The redistribution of electronic density in molecules after they absorb light is an ultrafast phenomenon of great importance involving quantum effects and molecular dynamics.
The ability to measure the electron and charge transfer dynamics with extreme temporal resolution not only provides a fundamental understanding of the physical mechanisms behind these processes, but also offers unique insights into how to engineer the chemical and structural properties of the molecule to control or enhance them.
Ultrashort ultraviolet pulses from high-order harmonic sources or free electron laser facilities stand as powerful tools for initiating and observing the response of molecules to photoionization, on timescales ranging from the femtosecond (10-15 seconds) down to the attosecond (10-18 seconds). Despite many advancements in these techniques, a detailed understanding of the initial steps of electron and charge transfer after prompt photoionization is not yet available.
In a study published in Nature Chemistry, researchers at Politecnico di Milano, Madrid Institute for Advanced Studies in Nanoscience (IMDEA Nanociencia), Autonomous University and Complutense University of Madrid unveil new insights into the ultrafast dynamics of molecular systems using attosecond extreme-ultraviolet pulses.
This pioneering work offers a fresh perspective on the complex interplay between electrons and nuclei in donor-acceptor molecules, significantly advancing our understanding of chemical processes at the most fundamental level.

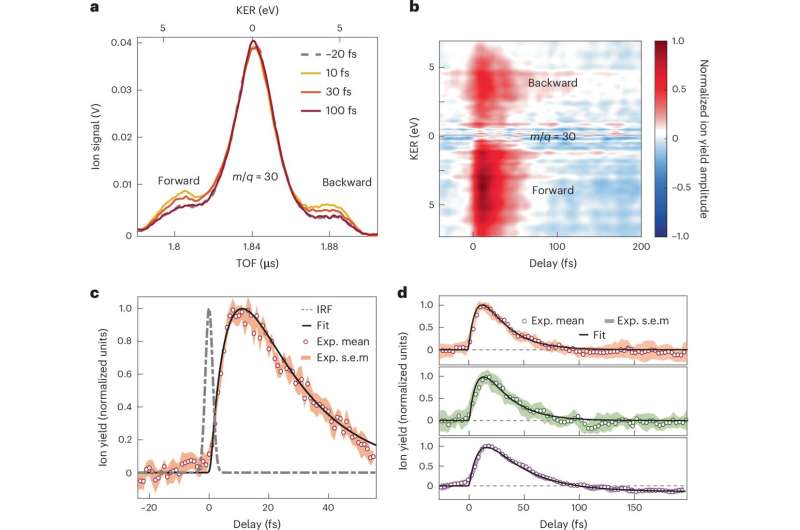
By exposing nitroaniline molecules to attosecond pulses, the research team has been able to observe and analyze the earliest stages of charge transfer with unprecedented precision. This study employs a combination of cutting-edge techniques, including attosecond extreme-ultraviolet-pump/few-femtoseconds infrared-probe spectroscopy and advanced many-body quantum chemistry calculations, to capture the dynamics of these rapid processes.
Precise temporal information on the various steps of the electron and charge transfer process have been thoroughly addressed. Key findings from the research reveal that electron transfer from the electron donor amino group occurs within less than 10 femtoseconds, driven by a synchronized movement of nuclei and electrons.
This is followed by a relaxation process that unfolds over a sub-30-femtosecond timescale, as the nuclear wave packet spreads in the excited electronic states of the molecular cation. These discoveries offer valuable new insights into how electron-nuclear coupling influences electron donor-acceptor systems in response to photoionization.
The results reported here unveil the times required to transfer charge from an electron donor unit to the adjacent chemical bond connecting that unit with a benzene ring, and for the concomitant required structural changes that occur. The authors believe that these experimental and theoretical findings pave the way to a better understanding of the textbook diagrams and concepts used to qualitatively predict charge migration in organic molecules.
This study not only sheds light on the intricacies of molecular dynamics but also sets the stage for future research in the field towards advancements in both theoretical understanding and practical applications of attosecond science.
More information:
Federico Vismarra et al. Few-femtosecond electron transfer dynamics in photoionized donor–π–acceptor molecules. Nature Chemistry (2024). DOI: 10.1038/s41557-024-01620-y , www.nature.com/articles/s41557-024-01620-y
Provided by
IMDEA Nanociencia
Citation:
Ultra-high speed camera for molecules: Attosecond spectroscopy captures electron transfer dynamics (2024, September 25)
retrieved 25 September 2024
from https://phys.org/news/2024-09-ultra-high-camera-molecules-attosecond.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.